About CBD
The discovery of CBD dates to 1940, when chemist Roger Adams extracted the CBD molecule from cannabis plants. The next big advance happened in 1963, when Raphael Mechoulam was able to describe the structure of CBD, leading him to be dubbed as the “godfather of cannabis”.
Once the structure was discovered, it paved the way for understanding more about the molecule and its activity on the human body. The structure of THC was discovered a year later and allowed scientists to make the link between THC and its euphoric, psychoactive effects, and the distinction between that and CBD. CBD was found to have numerous therapeutic effects on the body, to understand how it works, it is important to understand the endocannabinoid system (ECS).
The Endocannabinoid System
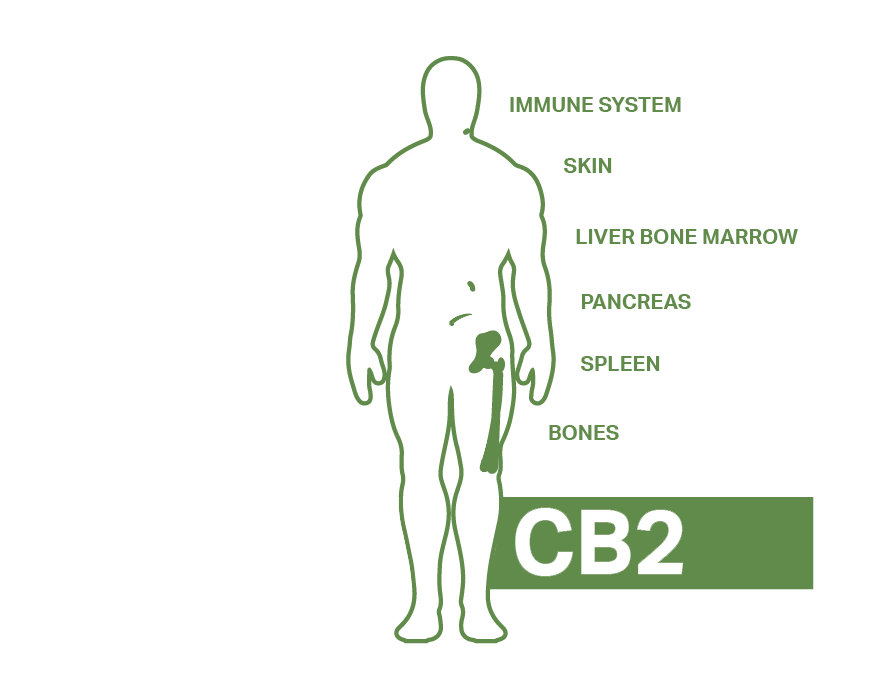 The ECS contains 3 key components, endocannabinoids, cannabinoid receptors and enzymes. Endocannabinoids are cannabinoids like CBD and THC but made by the body. The two we currently know of are anandamide (AEA) and 2-arachidonoylglyerol (2-AG). The receptor sites are found all over the body and are also activated when we ingest cannabis and are currently classified as either CB1 or CB2.
The ECS contains 3 key components, endocannabinoids, cannabinoid receptors and enzymes. Endocannabinoids are cannabinoids like CBD and THC but made by the body. The two we currently know of are anandamide (AEA) and 2-arachidonoylglyerol (2-AG). The receptor sites are found all over the body and are also activated when we ingest cannabis and are currently classified as either CB1 or CB2.
The ECS is one of the most influential systems in the human body, contributing to homeostasis or balance. The ECS influences functions as diverse as stress, appetite, energy, reproduction, pain, and sleep.
The endocannabinoid system is a complex regulatory system with broad functions, and found within all complex animals, from fish to humans. It regulates such diverse functions as memory, digestion, motor function, immune response and inflammation, appetite, pain, blood pressure, bone growth, and the protection of neural tissues, among others.
Unlike THC, CBD does not make you “high” and typically does not cause any negative effects. Experts are not completely sure how CBD interacts with the ECS. But they do know that it does not bind to CB1 or CB2 receptors the way THC does. Instead, many believe it works by preventing endocannabinoids from being broken down.
Different Types of Cannabinoids and Their Effects
113 different types of cannabinoids have been characterised to date.
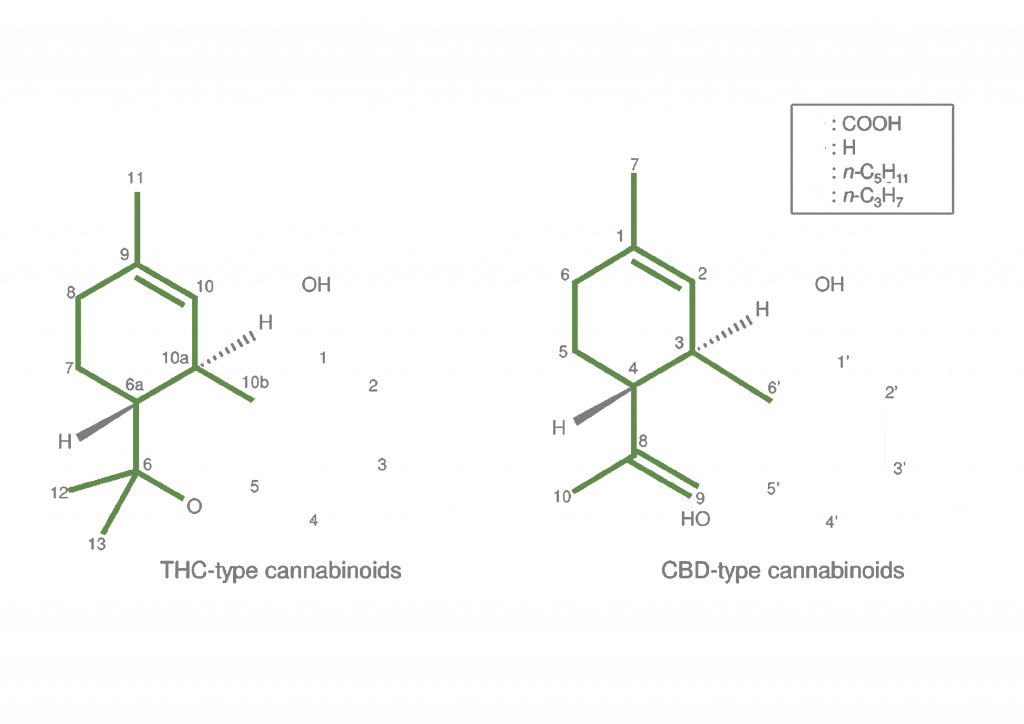
Below is a diagram illustrating the differences in THC-type and CBD-type cannabinoids. The variable regions are highlighted.
Whilst cannabinoids act via cannabinoid receptors, but they also affect the activities of many other receptors, ion channels and enzymes.
Cannabis does not directly make the most famous cannabinoids associated with the plant, THC and CBD. Instead, it synthesizes several cannabinoid acids. These cannabinoid acids must be “activated” (decarboxylated), usually by heat, to yield the compounds that most consumers are after (THC or CBD). But in addition to THCA and CBDA, there are number of related cannabinoid acids that can be produced by cannabis. These are:
CBGA (Cannabigerolic acid)
When harvested cannabis is heated, any remaining CBGA will decarboxylate into CBG. It’s thanks to this unique cannabinoid that all other medicinal benefits in cannabis are possible. In addition, CBGA has shown analgesic, antibacterial, anti-inflammatory, and anti-proliferative properties.
THCA (Δ9-tetrahydrocannabinolic acid)
THCA works to relieve inflammation, pain and is an ideal cannabinoid for treating symptoms of such conditions as arthritis, seizures. THCA is an effective neuroprotectant, so it is beneficial in the treatment of such conditions as multiple sclerosis, Alzheimer’s, and Parkinson’s disease.
CBDA (Cannabidiolic acid)
CBDa showed anti-inflammatory, antimicrobial and tumor-inhibiting properties and also suppressed nausea and vomiting,” according to the scientific studies conducted by DDr.
CBCA (Cannabichromenenic acid)
CBCA stems from the “mother cannabinoid” CBGA. A specialised enzyme known as cannabichromenic acid synthase (CBCAS) catalyses a reaction that turns CBGA into CBCA. The cannabinoid acid then acts as a precursor to two other cannabinoids.
CBGVA (Cannabigerovarinic acid)
As an important, if minor, component of most strains of Cannabis sativa, CBGV may also contribute to the entourage effect, so it would not be too surprising if this varin cannabinoid improved the effects of THC, CBD, or even CBG.
THCVA (Tetrahydrocanabivarinic acid)
THCV (tetrahydrocannabivarin) – not to be confused with psychoactive THC (tetrahydrocannabinol) – goes in a different direction, showing promise for sharpening the mind and suppressing appetite. Emerging animal research shows promising results for THCV’s effects on mental wellness – including antipsychotic effects in rats and neuroprotective effects in mice. In at least one study, participants with type 2 diabetes that used THCV reported improved blood sugar regulation. It also shows promise in stimulating bone growth.
CBDVA (Cannabidivarinic acid)
A non-psychoactive cannabinoid found in Cannabis that reportedly has anti-inflammatory properties.
CBCVA (Cannabichromevarinic acid)
CBC does not bind properly with CB1 receptors in the brain thus it does not produce any psychological effects. CBC is a highly effective painkiller. It offers antifungal and antibacterial properties, along with some antidepressant effects. It also has therapeutic effects in the treatment of diarrhoea and acne.
The CBD Timeline
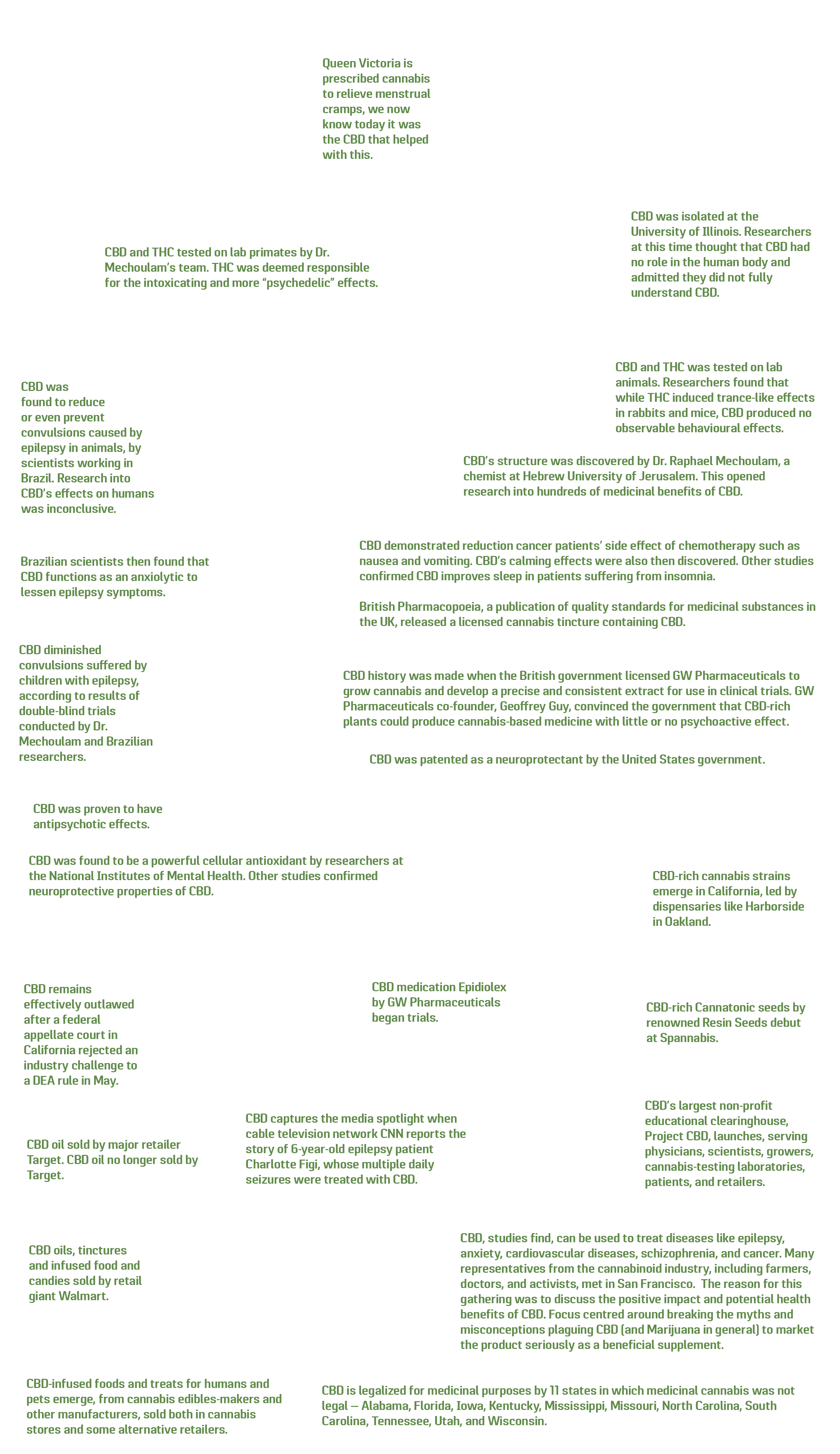
1800s: Queen Victoria is prescribed cannabis to relieve menstrual cramps, we now know today it was the CBD that helped with this.
1940: CBD was isolated at the University of Illinois. Researchers at this time thought that CBD had no role in the human body and admitted they did not fully understand CBD.
1946: CBD and THC was tested on lab animals. Researchers found that while THC induced trance-like effects in rabbits and mice, CBD produced no observable behavioural effects.
1964: CBD’s structure was discovered by Dr. Raphael Mechoulam, a chemist at Hebrew University of Jerusalem. This opened research into hundreds of medicinal benefits of CBD.
Late-1960’s: CBD and THC tested on lab primates by Dr. Mechoulam’s team. THC was deemed responsible for the intoxicating and more “psychedelic” effects.
1973: CBD was found to reduce or even prevent convulsions caused by epilepsy in animals, by scientists working in Brazil. Research into CBD’s effects on humans was inconclusive.
1974: Brazilian scientists then found that CBD functions as an anxiolytic to lessen epilepsy symptoms.
Mid-1970s: CBD demonstrated reduction cancer patients’ side effect of chemotherapy such as nausea and vomiting. CBD’s calming effects were also then discovered. Other studies confirmed CBD improves sleep in patients suffering from insomnia.
British Pharmacopoeia, a publication of quality standards for medicinal substances in the UK, released a licensed cannabis tincture containing CBD.
1980: CBD diminished convulsions suffered by children with epilepsy, according to results of double-blind trials conducted by Dr. Mechoulam and Brazilian researchers.
1982: CBD was proven to have antipsychotic effects.
Late 1990s: CBD was found to be a powerful cellular antioxidant by researchers at the National Institutes of Mental Health. Other studies confirmed neuroprotective properties of CBD.
1998: CBD history was made when the British government licensed GW Pharmaceuticals to grow cannabis and develop a precise and consistent extract for use in clinical trials. GW Pharmaceuticals co-founder, Geoffrey Guy, convinced the government that CBD-rich plants could produce cannabis-based medicine with little or no psychoactive effect.
1999: CBD medication Epidiolex by GW Pharmaceuticals began trials.
2003: CBD was patented as a neuroprotectant by the United States government (U.S. Patent #6,630,507)
2006: CBD-rich cannabis strains emerge in California, led by dispensaries like Harborside in Oakland.
2008: CBD-rich Cannatonic seeds by renowned Resin Seeds debut at Spannabis.
2010: CBD’s largest non-profit educational clearinghouse, Project CBD, launches, serving physicians, scientists, growers, cannabis-testing laboratories, patients, and retailers.
2011: CBD, studies find, can be used to treat diseases like epilepsy, anxiety, cardiovascular diseases, schizophrenia, and cancer. Many representatives from the cannabinoid industry, including farmers, doctors, and activists, met in San Francisco. The reason for this gathering was to discuss the positive impact and potential health benefits of CBD. Focus centred around breaking the myths and misconceptions plaguing CBD (and Marijuana in general) to market the product seriously as a beneficial supplement.
2013: CBD captures the media spotlight when cable television network CNN reports the story of 6-year-old epilepsy patient Charlotte Figi, whose multiple daily seizures were treated with CBD.
2014: CBD is legalized for medicinal purposes by 11 states in which medicinal cannabis was not legal — Alabama, Florida, Iowa, Kentucky, Mississippi, Missouri, North Carolina, South Carolina, Tennessee, Utah, and Wisconsin. The Agricultural Act of 2014 defined hemp as “cannabis containing less than 0.3% of THC”. There was zero mention within the legislation involving CBD! This meant both entrepreneurs and more established companies took it as a ‘green light’ that CBD was considered legalised. In a flash, CBD production grew exponentially.
2015: CBD-infused foods and treats for humans and pets emerge, from cannabis edibles-makers and other manufacturers, sold both in cannabis stores and some alternative retailers.
2016: CBD oils, tinctures and infused food and candies sold by retail giant Walmart.
2017: CBD oil sold by major retailer Target. CBD oil no longer sold by Target.
CBD, according to a statement by the U.S. Drug Enforcement Administration, “is being illegally produced and marketed in the United States in violation of two federal laws.”
2018: CBD remains effectively outlawed after a federal appellate court in California rejected an industry challenge to a DEA rule in May.
In the USA, The Agricultural Improvement Act of 2018, senator Mitch McConnell announced a complete federal legalisation of hemp. This also brought with it an influx of falsely labelled and low-quality CBD products, that remain largely unregulated in the ever-growing CBD market.
CBD medication Epidiolex by GW Pharmaceuticals receives FDA approval on June 25 for sales and use starting in 90 days.
CBD products are approved for sale in Canada under Cannabis Act details released June 27.
Benefits & Uses
- Relief of anxiety and depression
Preliminary scientific evidence has suggested that CBD may reduce anxiety, particularly those suffering from social anxiety. CBD has also shown promising results in animal studies for arthritis, noting reduction in inflammation and pain in the affected rat’s joints. CBD is a popular natural remedy for treating a variety of ailments that cause chronic pain, without causing negative side effects.
- Pain relief
Preclinical studies in animals using both pharmacological and genetic approaches have increased our understanding of the mechanisms of cannabinoid-induced analgesia and provided therapeutical strategies for treating pain in humans. The mechanisms of the analgesic effect of cannabinoids include inhibition of the release of neurotransmitters and neuropeptides from presynaptic nerve endings, modulation of postsynaptic neuron excitability, activation of descending inhibitory pain pathways, and reduction of neural inflammation.
- Alleviation of cancer related symptoms
There have been numerous studies into the use of CBD to alleviate cancer related symptoms and it has proven effective in the reduction of pain associated with cancer. It has also been shown to reduce chemotherapy side effects such as nausea and vomiting.
- Reduction of acne
CBD has proven to help multiple skin problems, including eczema, but has shown very effective results in reducing acne. Its anti-inflammatory effects as well as reduction of sebum production has rendered it a useful, safe, and natural way to treat acne.
- Sports recovery
Due to its analgesic and anti-inflammatory effects, CBD can be used to help alleviate muscle soreness and speed up recovery for athletic purposes.
- Addiction
It is unknown why CBD helps addicts in recovery; it could potentially be due to its anxiety reducing properties. It can help with nicotine, alcohol and even heroin withdrawal symptoms. There are schools of thought suggesting it may even help to rewire brain circuits associated with dependence.
- General wellbeing
Most prevalently, it can be used by anyone to help increase the feeling of wellbeing and general relaxation, calmness, and happiness.
Production of CBD Oil
One the hemp plants have successfully grown and matured, they are harvested and spread out evenly to ensure efficient drying. The dry hemp is milled to form a fine powder, often described as having a texture like coffee grounds. There are multiple methods that can be employed to extract CBD from this powder, but the ‘Gold Standard’ is the supercritical CO2 method of extraction. It should be noted that there are varying pros and cons for all the methods.
The processed material then undergoes extraction, to remove the useful cannabinoids and plant nutrients. The resultant extract is then combined with the carrier oil, which can vary between different manufacturers. Commonly used carrier oils are hemp seed oil, coconut oil, olive oil and MCT oil.
Different CBD manufacturers or brands put their own unique spin on each production step, which is why the many different products on the market can vary wildly in terms of final quality and the levels of cannabinoids they contain.
Market Growth
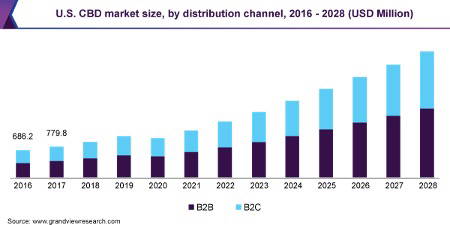
The valuation of the global cannabidiol (CBD) market was around $2.8 billion (USD) in 2020, with an expected compound annual growth rate of 21.2% between 2021 and 2028, it highlights a rapidly expanding commercial market! Due to the health benefits of CBD, the demand continues to rise and is the main factor driving growth within the sector. Additionally, the rising acceptance and understanding of products and government approvals (both the U.S and globally) are major factors expecting to boost production of CBD-infused products.
Product demand is also rising due to additional health applications attributed to CBD, which is being heavily advertised. Subsequently, the increase of dosage options now available for consumers such as edibles, analgesics, tinctures, oils and beyond are also driving the CBD consumer in the health market.
In terms of revenue share, retail pharmacies continue to dominate the overall market with 40.8% in 2019, with some predicting it will continue to grow at a lucrative pace over the coming years. Post-legalisation of CBD products in various countries and regions have led to broadening avenues for supplying CBD directly to consumers.
E-commerce of CBD has widely increased its market potential, enabling companies to expand their market worldwide to more inaccessible locations. In addition, big companies like Sephora and GNC have taken on brands or collaborated with brands to sell their CBD products.
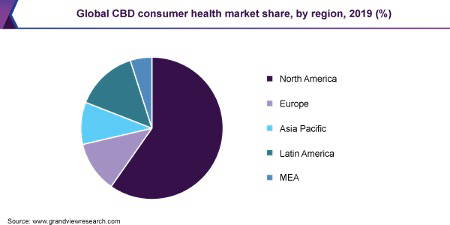
North America dominated the market for cannabidiol consumer health products with a revenue share of 59.8% in 2019. This stems from their higher concentration of CBD companies and relaxed laws regarding CBD products.
AS consumption increased, a rise in awareness about the health benefits of CBD-based food and health products increased. Introduction of the Farm Bill 2018, legalising hemp cultivation and manufacturing of hemp-derived products in the U.S. drove the demand increase in North America.
| Market size value in 2020 | USD 9.42 billion |
| Revenue forecast in 2027 | USD 43.8 billion |
| Growth Rate | CAGR of 24.6% from 2020 to 2027 |
Regulation
Importing CBD: No strict requirements apply for importing CBD into the UK provided THC is not detected* by the authorities at the border.
Selling CBD: Depends on product category: Cosmetics require Cosmetic Product Safety Report (CPSR). Vape products should comply with non-nicotine e-liquid regulation i.e., General Products Safety Directive: You do not currently require a ‘hemp license’ to sell CBD in the UK provided THC is not detected*.
* ‘Not detected’ means no THC at 0.01% as verified by accredited ISO lab.
CBD flowers: The sale of ‘CBD Flowers’ and buds is prohibited even if THC is below 0.2% and from EU approved origin.
CBD food/food supplements: As of 13th February 2020, new products on the market require a Novel Food application. Products on the UK market before 13th February 2020, require a validated submission or approved novel food application by 31st March 2020.
Applying for a license: Whether you wish to apply for novel food status or any other type of license relating to CBD or hemp requires significant capital, time, and resources.
NOTE: A soft stance on enforcement by the UK authorities is the reason why we see prohibited products such as CBD flowers and unlicensed CBD foods without novel food application openly sold in shops in the UK.
Selling CBD in other EU markets: Each Member State has their own laws on CBD, most of which are stricter than the UK. Expert advice is required to navigate local laws.
References
References:
[1] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4604171/ 2015
[2] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4851925/ 2016
[3] https://www.frontiersin.org/articles/10.3389/fphar.2018.01259/full 2018
Useful links:
https://www.grandviewresearch.com/industry-analysis/cannabidiol-consumer-health-market
https://www.greenmarketreport.com/2021-cbd-market-to-outpace-2020-per-new-brightfield-report/
https://www.cultbeauty.co.uk/trends/cannabis-beauty
https://www.themanual.com/food-and-drink/best-cbd-drinks/
https://www.leafly.com/news/category/cbd
https://www.boerejongens.com/cbd-in-sports/#
https://www.bikemag.com/blog/benefits-of-cbd-for-professional-action-sport-athletes/
https://www.sciencedaily.com/releases/2019/05/190521101450.htm
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7053164/
17/6/21: https://www.cbd-intel.com/uk-fsa-releases-initial-validated-novel-food-application-list/